www.showameraucanas.com
|
I have come across several posts recently asking questions about cross beak and its origin. Numerous scientific studies have been completed on the subject. I reviewed several of the scientific studies that are available on cross beak and summarized the results below.
W. Landauer reported that the frequency of eye abnormalities with cross beak present is greatly influenced by incubation temperatures (Landauer, 1937). Landauer carried out several test matings of affected stock and found it impossible to produce a true-breeding cross billed fowl, despite considerable inbreeding (Landauer, 1938). In 1941, Landauer identified a lethal mutation in fowl that produces a shortening of the upper beak and of the long bones of the extremities. He reported the mutation to be recessive, autosomal and semi-lethal. In the experiments he completed, most homozygous embryos died near the end of the incubation period, but about 13% of all homozygotes hatched. The degree of shortening of the upper beak and of the long bones was variable. In roughly 50% of “short upper beak” chicks the beak became normal during growth. In the remaining 50%, a cross beak developed. (Landauer, 1941). Hutt (1949) and Pflugfelder (1961) reported that there are non-hereditary deformed beaks associated with unilateral microphthalmia or anophthalmia in chick embryos. Hutt noted in his research findings that most cross beak cases that occur because of these conditions are never seen because the chicks die during the later stages of incubation. Hutt reported this abnormality most likely results from an accident in development, sometimes induced by an unfavorable environment. (Hutt, 1949). Pflugfelder set up matings of 12 hens and 5 cocks affected with unilateral microphthalmia or anophthalmia to determine if the deformed beak that occurs with these conditions is hereditary. All the offspring obtained from his experiment were normal and he concluded that this type of cross beak was not genetic. (Pflugfelder, 1961). Moriyuki Watanabe completed a small-scale study to explore non-hereditary causes of cross beak. He found that variations in incubator temperature due to interruptions in electrical current were causal of the condition. (Watanabe, 1966). A breeding trial performed on Appenzeller Barthuhn chickens, a breed believed to have a genetic predisposition to cross beak, showed significantly higher prevalence of offspring with deformed beaks from the mating of parent stock with cross beak present compared to the mating of non-affected parents. The mating of parent stock with cross beak resulted in 67 (80.7%) offspring with normal beaks and 13 (15.7%) with cross beak. The mating of parent stock with normal beaks resulted in 95 (93.1%) offspring having normal beaks and 3 (2.9%) having cross beak. The findings elude to a possible hereditary cause of cross beak, but variations in the phenotype and inconclusive molecular genetic results indicates the need for additional research. (Joller et al., 2018). A scientific study completed in 2019 on Huiyang bearded chickens found that expression levels of bone morphogenetic protein 4 (MBP4) in the craniofacial bones was associated with the occurrence of cross beak. The expression of MBP4 was highest in chicks that had severe cross beak followed in succession by those with moderate and minimal cross beak. This study provided insight into the potential role of BMP4 in the development of this congenital abnormality. (Hong et al., 2019). A scientific study was completed on congenital anomalies by A. Azizpour in 2019. A total of 1,796,863 broiler chicks were examined during this study. 1,740,832 chicks were labeled as normal at hatch and 56,031 chicks were identified as having a congenital anomaly. Cross beak was identified in 27.43% of the chicks that had a congenital anomaly present. Azizpour reported that genetics and management factors were causal of cross beak. (Azizpour, 2019). In summary, a few of the factors that have been found to be associated with and/or causal of cross beak are incubation temperatures and fluctuations, hereditary factors, accidents in development, bone morphogenetic protein 4 and management factors. References: Azizpour, Aidin. (2019). A Study on Congenital Anomalies in Hatched Broiler Chickens at the End of the Incubation Period. 10.22092/vj.2018.123598.1505. Hong, Y.; Pang, Y.; Zhao, H.; Chen, S.; Tan, S.; Xiang, H.; Yu, H.; Li, H. The Morphology of Cross-Beaks and BMP4 Gene Expression in Huiyang Bearded Chickens. Animals 2019, 9, 1143. Hutt, F.B. 1949. Genetics of the Fowl. 1st ed. pp. 43-44. McGraw-Hill Book Comp. INC. Joller, Sara & Bertschinger, Flurina & Kump, Erwin & Spiri, Astrid & Rotz, Alois & Schweizer-Gorgas, Daniela & Drögemüller, Cord & Flury, Christine. (2018). Crossed Beaks in a Local Swiss Chicken Breed. BMC Veterinary Research. 14. 10.1186/s12917-018-1398-z. Landauer W. A Semi-lethal Mutation in Fowl Affecting Length of the Upper Beak and of the Long Bones. Genetics. 1941;26(4):426-439. Landauer, W. Notes on Cross-beak in Fowl. Journ. of Genetics 37, 51–68 (1938). https://doi.org/10.1007/BF02982143 PFLUGFELDER, 0. 1961. Nichterblich Kreuzschnabelbildungen beim Haushuhn. Roux' Arch. Entw. 152: 655-661. Watanabe, Moriyuki. 1966. Non-hereditary Crooked Beaks in Poultry. Department of Animal Husbandry, Faculty of Fisheries and Animal Husbandry, Hiroshima University. 6: 339-34.
0 Comments
A few folks have emailed me to ask about the potential results of using a blue Ameraucana in a self blue (formerly known as lavender) Ameraucana pen. I wanted to take a moment to discuss the two color varieties and what results one may encounter when crossing a blue Ameraucana with a self blue Ameraucana. This is merely an educational post and is not meant to discourage anyone from pursing projects that they may enjoy. However, when asked about the subject I discourage folks from using a blue Ameraucana in a self blue Ameraucana pen as the offspring produced do not adhere to the standard. Let’s explore the differences in the blue gene (Bl) and the lavender gene (lav).
The blue gene (Bl) is a diluting gene. It is known as an autosomal incomplete dominant gene. Chickens have 39 pairs of chromosomes. They have two categories of chromosomes known as sex chromosomes and autosomes. An autosome is simply defined as a chromosome that is not a sex chromosome. Genes are contained within these chromosomes. Each parent contributes one allele to form a particular gene in a diploid organism. Incomplete dominance means that one allele is not completely expressed (or dominant) over the allele that it is paired with. This results in a phenotype that is a combination of both alleles. This explains why blue chicks produced in a blue, black and splash breeding pen are not all the exact same shade of blue. Some may be lighter in color and others may be darker in color due to the incomplete dominance. In its heterozygous form (one copy of the Bl gene, known as Bl/bl+), black feathers are diluted to create blue plumage. In its homozygous form (two copies of the Bl gene, known as Bl/Bl), black feathers are diluted to create splash plumage. The genotype for the wild type (the non-mutated version which is black) is bl+/bl+. The American Poultry Association standard of perfection calls for the plumage of a blue Ameraucana to be an “even shade of clear bluish slate with each feather distinctly laced with black.” While there are a few conflicting studies out there, multiple scientific studies have found that there are three genes involved in creating the black single lacing on a blue Ameraucana. Those three genes are the Pattern gene (Pg), Melanotic gene (MI) and Columbian gene (Co). The Pattern gene is responsible for creating patterns on plumage. It organizes black pigment concentrically. The Melanotic gene is a black intensifier. It enhances and moves black pigment to the outer border of the feather. This makes the outer border black and double lacing is created (Pg+Ml). By adding Columbian (Co), which is an eumelanin restrictor, the inner laces are taken away and single lacing is created (Pg+Ml+Co). Blue cockerels are also darker in the hackle, saddle and wingbows as a result of their sexually dimorphic plumage. I spoke with one of my mentors who has written several poultry genetics books about what creates the darker hackle, saddle and wingbows on blue cockerels and he confirmed that it is the result of additional melanizers. He stated there are around two dozen melanizers in poultry, many of which are unnamed. When blues are used in a self blue breeding pen, the cockerels have darker hackle, saddle, and wingbows. This does not adhere to the standard which calls for “no contrast in color between any of the sections.” The lavender gene (lav) is an autosomal recessive gene. It dilutes eumelanin and pheomelanin. The lavender gene has not been shown to be epistatic to other diluters and contributes in further dilution of plumage color. Therefore, when a self blue Ameraucana that is carrying the blue gene is crossed with a self blue Ameraucana, a more diluted plumage color is created as a result of the two diluting genes. This very diluted plumage color does not adhere to the standard as the standard calls for the plumage of a self blue Ameraucana to be “a MEDIUM shade of clear blue).” Furthermore, if you mate a self blue bird carrying the blue gene with another self blue bird that is carrying the blue gene, an even further diluted plumage color can be produced with splash spots present. Curiously, you may also notice that many of the birds in these crosses have dark feather shafts as well and the standard states that self blues should be “free from shaftiness (see the attached example photo).” In closing, three reasons to be cautious about using a blue Ameraucana in a self blue Ameraucana pen are: 1) The combination of the two diluting genes (lav and Bl) results in a very diluted plumage color that does not adhere to the standard description. The standard calls for the plumage of a self blue Ameraucana to be a “medium shade of clear blue.” 2) The Pg, MI and Co genes that a blue Ameraucana has can result in partial or incomplete lacing in offspring. This does not adhere to the standard description. The standard states that the plumage should be “free from lacing, shaftiness, mealiness and messiness.” 3) Melanizers that the blue Ameraucanas carry result in sexually dimorphic plumage on cockerels (darker hackles, saddles and wingbows) which does not adhere to the standard description. The standard states that there should be “no contrast in color between any of the sections.” Do you have a breeding or genetic question related to Ameraucanas? Email me at [email protected] and I may create an educational post about it! Are you interested in working with Ameraucanas and breeding them towards the standard? Join the Ameraucana Breeders Club at www.ameraucanabreedersclub.org! To breed Ameraucanas to standard and to be aware of all defects and disqualifications, you will need a copy of the APA SOP book. To buy an American Poultry Association Standard of Perfection book, visit the following link: http://amerpoultryassn.com/store.htm *This post is under copyright and cannot be used or published without the consent of the author. Poultry Science Volume 70, Issue 1, 1 January 1991, Pages 1-5. W. C. Carefoot (1992) Inheritance of the lace‐tailed laced plumage pattern of the sebright bantam, British Poultry Science, 33:2, 297-302, DOI: 10.1080/00071669208417468 W. C. Carefoot (1988) Inheritance of the laced plumage pattern of the blue Andalusian bantam, British Poultry Science, 29:1, 175-178, DOI: 10.1080/00071668808417040 W. C. Carefoot (1986) Laced and double‐laced plumage pattern phenotypes of the domestic fowl, British Poultry Science, 27:1, 93-96, DOI: 10.1080/00071668608416858 Muffs and beard (Mb) is known as an autosomal incomplete dominant trait. It is an observable physical characteristic involving elongated feathers that form on the side of the face and below the beak.
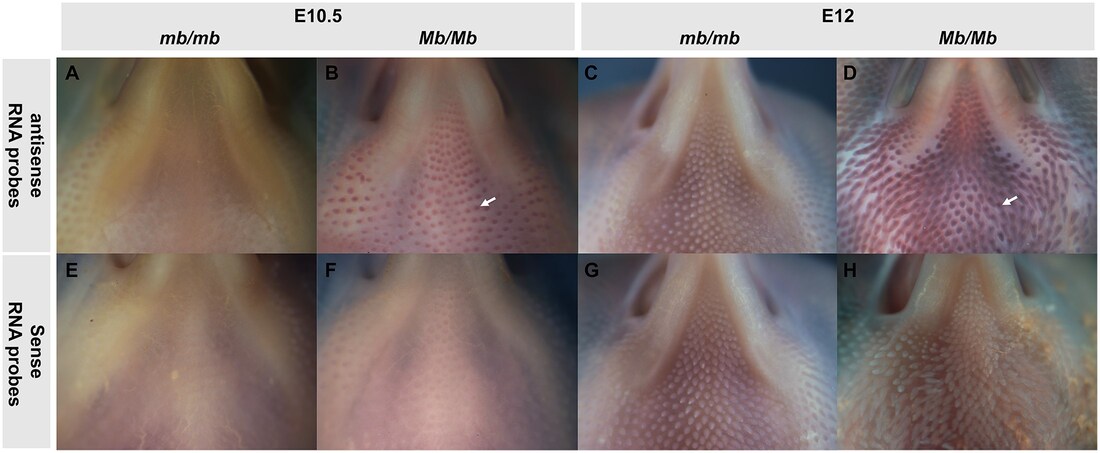
First, let’s break things down and explore the meaning of an autosomal incomplete dominant trait. Chickens have 39 pairs of chromosomes. They have two categories of chromosomes known as sex chromosomes and autosomes. An autosome is simply defined as a chromosome that is not a sex chromosome. Genes are contained within these chromosomes. Each parent contributes one allele to form a particular gene in a diploid organism. Incomplete dominance means that one allele is not completely expressed over the allele that it is paired with. This results in a phenotype that is a combination of the phenotypes of both alleles. There are three genotypes for the Mb locus regulating the Mb trait: 1) Mb/Mb homozygous (carrying two copies of the gene and exhibiting a full muff/beard) 2) Mb/mb heterozygous (carrying one copy of the gene and exhibiting a partial muff and beard) 3) mb/mb wild-type homozygous (not carrying any copies of the gene and what many refer to as clean-faced) So how does the muffs and beard (Mb) trait affect our Ameraucana breeding programs? Using a punnett square, we can predict the genotypes of a particular cross. If you mate two homozygous Mb/Mb birds together, all resulting offspring will have two copies of the Mb gene and full muffs and beard. If you mate two Mb/mb heterozygous birds together, 25% of the offspring will be homozygous Mb/Mb and have full muffs and beard, 50% will be heterozygous Mb/mb and have partial muffs and beard, and 25% will be homozygous wild-type mb/mb and not have muffs or a beard. If you mate a Mb/Mb homozygous bird with a Mb/mb heterozygous bird, 50% of the offspring will have full muffs and beard and 50% will have partial muffs and beard. Mb birds are born with muffs and beard, and the remarkable differences of Mb/Mb, Mb/mb, and mb/mb birds can be witnessed during embryonic development. I have attached a photo from a study that shows HOXB8 expression is increased considerably in the facial skin of developing Mb/Mb embryos and postnatal chicks (Guo et al., 2016). This study also found that the Mb allele that causes the Mb phenotype is “a derived allele where a complex structural variation (SV) on GGA27 leads to an altered expression of the gene HOXB8. It is a structural mutation resulting from the duplication of three regions on chicken chromosome 27 (GGA27). The three duplication regions are located around 1.70 Mb (CNV1), 3.58 Mb (CNV2), and 4.47 Mb (CNV3) on GGA27, respectively (Guo et al., 2016).” lt is important to note that several studies have identified that wattles are absent or small when Mb is present (RG, S 1990). Many researchers speculate that the Mb trait is highly associated with the Wattles locus and that there are underlying complex interactions between Mb and Wattles. Hopefully this post will help you as you are assessing your Ameraucana chicks and breeding age birds for the Mb phenotype and setting up breeding pens. Good luck with your flock! Are you interested in learning more about Ameraucanas and breeding them towards the standard of perfection? Join the Ameraucana Breeders Club at ameraucanabreedersclub.org. To purchase an American Poultry Association standard of perfection book, visit the following link: http://www.amerpoultryassn.com/store.htm Guo Y, Gu X, Sheng Z, Wang Y, Luo C, Liu R, et al. (2016) A Complex Structural Variation on Chromosome 27 Leads to the Ectopic Expression of HOXB8 and the Muffs and Beard Phenotype in Chickens. PLoS Genet 12(6): e1006071. RG S (1990) Mutations and major variants of plumage and skin in chickens. In: Crawford RD, editor Poultry Breeding and Genetics Amsterdam, Netherlands: Elsevier: 169–208. When studying the American Poultry Association standard of perfection for a Wheaten Ameraucana cockerel/cockbird, we find the following:
1. The saddle should be “lustrous, light orange, free from dark feathers.” The saddle feathers are found “at the rear of the back extending to the juncture of back and tail of a male fowl, covered with long pointed fingers known as saddle feathers.” 2. The main tail should be “black with lustrous greenish gloss.” The main tail feathers are “the straight, stiff long feathers of the tail located under and between the coverts and sickles of the male.” 3. Lesser sickles should be “lustrous, greenish black with reddish cast in shafting.” The lesser sickles are “the long curved feathers of the male chicken tail, exclusive of the top two longest main-sickles, which hang to the side of and cover most if not all of the main tail.” Shafting is “a color characteristic where the shaft of a feather is either lighter or darker than the color of the web.” So in this case, we want to look for a reddish cast on the shaft of the lesser sickle feathers. *Here are some notes on cutting for defects: 1. Regarding the main tail feathers, foreign color results in a 1/2 pt deduction per feather that it is present. 2. Regarding the sickle feathers, foreign color results in a 1 pt deduction per feather that it is present. These are a few points to keep in mind when assessing your wheaten cockerels and cockbirds. To view the full standard, you can purchase an American Poultry Association Standard of Perfection book at the following link: http://www.amerpoultryassn.com/store.htm The blue eggshell of an Ameraucana fowl is one of the attributes that draws people to the Ameraucana breed. In this post, the biochemical and physiological characteristics of the blue egg shell are reviewed. Numerous studies and scientific advances have occurred over the years which have gradually increased our knowledge of the blue-green pigments that create the characteristic blue egg shell of an Ameraucana fowl. It is important to note that the pigment has been renamed several times over the course of the past century, which may lead to some confusion if you research the topic. In the 1800s, and in several studies completed since then, the pigment that causes blue eggs is referred to as biliverdin. In the late 1800s H.C. Sorby completed a scientific study and referred to the same pigment as Oocyan (Sorby, 1875). As scientific advances have occurred, more in depth studies on eggshell pigments have been completed. The blue-green pigments that create blue eggshells are presently identified as Biliverdin-IX and Zinc Biliverdin Chelate.
Friedrich Tiedemann (1781-1861), a german physiologist and anatomist, was one of the first individuals to study the blue-green pigment that we now refer to as biliverdin. He created the now famous gmelin reaction (a diagnostic color test) which was able to identify bilirubin in bile. Bilirubin is a derivative of biliverdin (Tiedemann, 1814). In 1858, W. Wicke presented what is considered to be the first known chemical essay on egg color pigments. Wicke treated egg shells with hydrochloric acid, water and boiling alcohol. During the process, he collected and identified a bluish green pigment that he declared to be biliverdin (Wicke, 1858). In 1875, H.C. Sorby published "Proceedings of the Zoological Study," which is considered to be the first scientifically based publication on the pigments present in blue eggs. He completed spectrum analysis of colored egg shells and identified seven substances present in various egg shells. Those substances were labeled: 1) Oorhodeine, 2) Oocyan, 3) Banded Oocyan, 4) Yellow Ooxanthine, 5) Rufous Ooxanthine, 6) Lichnoxanthine, and 7) a substance imperfectly distinguished (Sorby, 1875). Oocyan ("oo" = egg, "cyan" = blue) eventually became known as biliverdin within the scientific community. The pigment Sorby labeled as Oorhodeine eventually became known as Protoporphyrin IX. Protoporphyrin IX has been identified as the pigment that creates brown egg shell color. R.C. Punnett was the first individual to classify the pigment found on brown egg shells as protoporphyrin. Punnett also discovered that the blue-green eggshell color in chickens was produced by an autosomal dominant gene called Oocyan. Punnet noted in his scientific study that eggs laid by Oocyan homozygotes were a darker blue than those laid by heterozygotes (Punnett, 1933). Kennedy and Vevers completed a study in 1973 and found that blue eggs laid by Araucanas contained Biliverdin-IX, Zinc Biliverdin Chelate, and Protoporphyrin-IX (Kennedy, et al., 1973). They completed an additional study on 108 Araucana fowl in 1976 and confirmed that only the pigments Biliverdin-IX and Zinc Biliverdin Chelate could be detected in blue eggs and were causal of the blue coloring. They confirmed in their studies that brown egg shells contained large amounts of the pigment Protoporphyrin-IX (Kennedy, et al., 1976). The following is an excerpt of the findings they made during the study: “Eggshells from 108 species were examined for appearance and pigment content. Principal pigments found were protoporphyrin, biliverdin IXα and its zinc chelate. 49 species had protoporphyrin only, 2 had biliverdin only, 33 had protoporphyrin and biliverdin, 17 had all 3 pigments, one species had biliverdin and its zinc chelate, one had protoporphyrin and biliverdin zinc chelate and five species had no pigment. Biliverdin zinc chelate was never found alone (Kennedy et al., 1976).” In pullets and hens that lay an egg on the olive green or khaki spectrum, a coating of the pigment Protoporphyrin-IX has been added to the egg during the final hours of the egg being inside the uterus. The Protoporphyrin-IX pigment does not penetrate every single layer of an egg shell. So, if you crack open an olive egg, you will likely find that the interior of the egg shell is still blue. Biliverdin, on the other hand, passes through all eight layers of the egg shell. If you crack open a blue Ameraucana egg shell, you will find that the interior of the egg shell is also blue (Wang et al., 2007). The higher the concentration of Biliverdin within the uterus of a hen, the bluer the egg will be. The higher the concentration of Protoporphyrin-IX within the uterus of a hen, the more olive or brown an egg will be (Wang et al., 2009). This information was confirmed in the study below. “The quantity of biliverdin of Dongxiang blue-shelled chickens was much more than Dongxiang brown-shelled chickens, whereas the quantity of protoporphyrin of Dongxiang blue-shelled chickens was only about half that of Dongxiang brown-shelled chickens, which hinted at the probable different transformation from precursor to biliverdin or protoporphyrin between blue-shelled chickens and brown-shelled chickens (Wang et al., 2009).” If you click the link below, there is a table in the published study that lists the pigments that were studied and the correlation between the amount of each pigment and the egg shell color produced. https://academic.oup.com/ps/article/88/8/1735/2886225 Interestingly, scientific research has shown that Biliverdin is created within the shell gland and then is deposited on the eggshell roughly 3-4 hours before ovi-position (Wang, et al., 2009). A study was done in 2010 to map the blue egg allele locus. The blue egg allele is identified by the letter “O.” A total of 98 blue egg laying hens were studied, and the map was identified as (TTA) n –(TG) n –A–O–(tg) n. The study also found that the “O locus was located between theA and (tg) nloci, that is, Chr1:67,296,991-69,140,571, which is the first genomic sequence interval to be established for the blue eggshell gene (Wang et al., 2010).” A research study completed in 2013 found that a blue egg occurs due to the expression of the SLCO1B3 gene. The SLCO1B3 gene is a part of the organic anion transporting polypeptide (OATP) family. The OATPs function as membrane transporters, and have been proven to transport bile products such as Biliverdin. An EAV-HP insertion in the 5′ flanking region of the SLCO1B3 gene was found to be connected to the blue egg phenotype (Wang et al., 2013). Many folks are unaware that the color varieties of Ameraucanas were initially created by crossing birds of varying backgrounds and selecting for specific attributes. Blue egg color was one part of that equation. The attributes that were selected for were eventually used to create the breed standard for each of the approved Ameraucana color varieties. The APA standard calls for Ameraucanas to have several other important attributes as well, such as a pea comb, reddish bay eye color, a beard, muffs, slate shanks, etc. It is important to remember that there are no perfect birds out there. Breeding birds towards the standard of perfection is all about balance. Don't become so lost in any one feature or attribute that you forget about the other aspects of the standard. Narrow your birds down carefully each season and keep the ones that most closely adhere to the standard. If you need to improve egg color in your Ameraucana flock, toe punch chicks that hatch from the bluest eggs. If the chicks grow up to be pullets, you can monitor them and hopefully they will lay an equally blue egg. If the chicks grow up to be cockerels, you will have foreknowledge that they hatched from a very nicely colored blue egg and are most likely carrying good blue egg genes. Good luck with your Ameraucana flock! I included a photo of an Ameraucana eggshell below to show how the Biliverdin-IX and Zinc Biliverdin Chelate pigments pass through the entire egg shell. -L. Helton REFERENCES Kennedy, G. Y., and H. G. Vevers. 1976. A survey of avian eggshell pigments. Comp. Biochem. Physiol. 55B:117–123 Kennedy, G. Y., and H. G. Vevers. 1973. Eggshell pigments of the Araucano fowl. Comp. Biochem. Physiol. 44B:11–25. Punnett, C. 1933. Genetic Studies in Poultry. J. Genet. 27:465-470. Sorby, H. C. (1875). On the colouring-matters of the shells of birds' eggs. Proceedings of the Zoological Society of London 1875: 351–365 Tiedemann, F. Journal de Pharmacie, Tome XXIII, p. 109. Wang Z, Qu L, Yao J, Yang X, Li G, Zhang Y, et al. (2013) An EAV-HPInsertion in 5′ Flanking Region of SLCO1B3 Causes Blue Eggshell in the Chicken. PLoS Genet 9(1): e1003183. https://doi.org/10.1371/journal.pgen.1003183 Wang, X.T., C. J. Zhao, J. Y. Li, G. Y. Xu, L. S. Lian, C. X. Wu, X. M. Deng, Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs, Poultry Science, Volume 88, Issue 8, August 2009, Pages 1735–1739,https://doi.org/10.3382/ps.2008-00434 Wang, X.T., J.-R. Bai, C.-J. Zhao, H. Zhang, H.-G. Bao, G.-Y. Xu, J.-Y. Li, Dr L.-S. Lian, C.-X. Wu & Dr X.-M. Deng (2010) Localisation of the genomic sequence interval for the blue eggshell gene using an F2 resource population of Dongxiang chickens, British Poultry Science, 51:4, 507-509, DOI: 10.1080/00071668.2010.502520 Wang, X.T., Deng, C.-J. Zhao, J.-Y. Li, G.-Y. Xu, L.-S. Lian, C.-X. Wu, Study of the Deposition Process of Eggshell Pigments Using an Improved Dissolution Method, Poultry Science, Volume 86, Issue 10, October 2007, Pages 2236–2238, Wicke, W. (1858). Uber des pigment in den eischalen der vogel. Naumannta 8: 393–397. Zhao, R., G.-Y. Xu, Z.-Z. Liu, J.-Y. Li, N. Yang, A study on eggshell pigmentation: biliverdin in blue-shelled chickens, Poultry Science, Volume 85, Issue 3, March 2006, Pages 546–549, https://doi.org/10.1093/ps/85.3.546 The genes and modifiers that affect shank color in poultry are not quite as straightforward as one would presume. As advances in science occur and more research studies take place, we are granted the opportunity to learn more about the genes, loci, and various modifiers that affect shank color. However, it is important to note that there are still many “unknowns.” Hopefully in time as more research is completed, we will continue to increase our knowledge about the genes, alleles and modifiers that affect shank color. Many times a year, I see posts on Ameraucana forums and groups regarding the shank color of wheaten, blue wheaten, and splash wheaten Ameraucanas. Posts like “Why do my day old wheaten and blue wheaten chicks not have slate shanks?” or “Why am I seeing variations in the amount of pigmentation on my wheaten and blue wheaten Ameraucana shanks?” or “Why do the wheaten and blue wheaten Ameraucanas that I bought not have really dark slate legs like my black Ameraucanas?” Many times exhibitors and breeders do not take the time to educate themselves regarding the underlying genetics of shank color and the genes and modifiers that affect it. The APA standard of perfection calls for Ameraucanas to have white skin and slate shanks. It is important to note that the APA standard was written for Ameraucanas that have reached sexual maturity, not for day olds. As it stands now, there are (*at least) three factors known to affect shank color in wheaten, blue wheaten and splash wheaten Ameraucanas. They are: 1) Skin color 2) Id gene 3) eWh allele First, let’s talk about skin color. The gene that is responsible for white skin is known as W+. It is dominant and wild type. The yellow skin gene is known as “w” and is recessive to W+. Both W+ and w can affect the epidermal layer (outer layer) of skin on Ameraucana shanks. To double check the skin color of your birds, take a close look at the foot pads on your Ameraucanas. They should be white. You can also take a look at other areas such as their beaks. Yellow, willow or green tints in these areas are indicative of yellow skin. Yellow skin is a disqualification. Next, let’s talk about the Id gene. There are actually several Id alleles, including Id, id^a, id^c, id^M, and id+ which are known to effect dermal melanin expression. Today I will just focus on Id and id+ in regards to their impact on wheaten, blue wheaten, and splash wheaten shank color. Id (which is the absence of black pigment) and id+ (which is the presence of black pigment) affect the dermal (underneath) layer of skin on Ameraucanas. They are found on the sex chromosome and are sex linked. Males have two copies and females have one copy. Dermal melanin comes in slowly on eWh chicks and can take anywhere from four to sixteen weeks to come in. This is why wheaten and blue wheaten chicks are born with flesh colored feet that gradually turn slate with age. Last, let’s talk about the eWh allele. The e locus alleles produce the base colors and patterns that color varieties are built upon. Some of the most common e locus alleles are: E (Extended Black) E^R (Birchen) e^Wh (Dominant Wheaten) e+ (Wild Type) e^b (Brown) eWh, which is the allele that wheaten and blue wheaten Ameraucana chicks should be based on, is known to inhibit the expression of dermal melanin. It can reduce and even hide the expression of it. This is one reason why you can’t always compare the shanks of your wheaten Ameraucanas (which are based on eWh) to for example your black Ameraucanas (which should be based on E) as they are based on completely different E locus alleles. E is known to extend eumelanin and enhance dermal melanin. I hope this post has given you a preview of some of the genes, alleles, and modifiers that can affect shank color in wheaten, blue wheaten and splash wheaten Ameraucanas. When narrowing down your birds and selecting breeders, I recommend selecting for dark shanks. While there are some variations in opinion among members of the scientific community, these three factors have been studied extensively and have continually been proven to impact shank color. Have a good day from Once In a Blue Moon. Shank color on day old wheaten, blue wheaten and splash wheaten Ameraucanas: Shank color of sexually mature wheaten and blue wheaten Ameraucanas:  A pullet with yellow skin, which is a disqualification for Ameraucanas, is pictured below on the left. A pullet with white skin, which is what the SOP calls for, is pictured on the right.
|
AuthorI enjoy spending time outdoors, studying poultry genetics and working with show quality Ameraucanas. All information shared on my blog is under copyright. Archives
July 2020
Categories |